|
CRO - CDMO
|
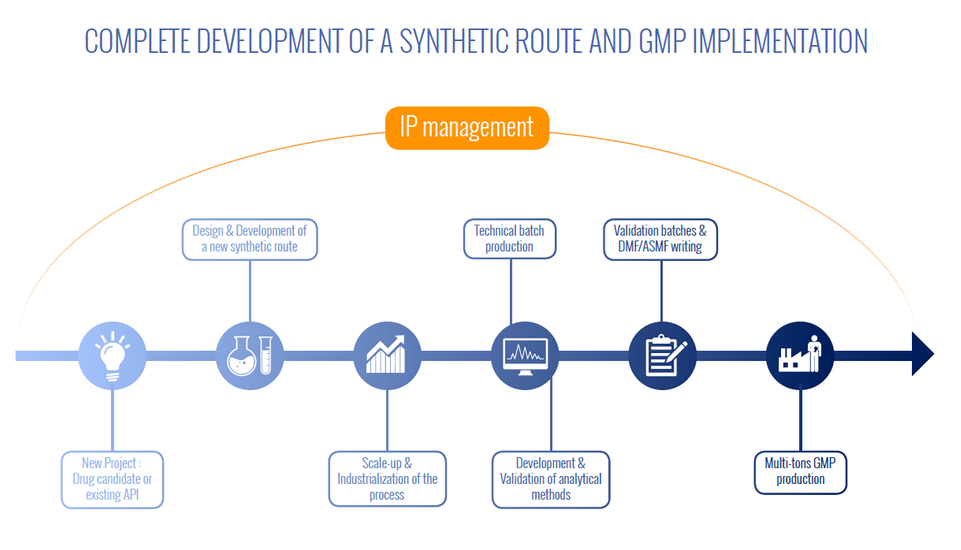
Our custom synthesis business has been designed to integrate completely all the phases of chemical development: design of a novel synthetic pathway accompanied by supply of a few grams, provision of pilot batches following development of the industrial procedure and GMP production of several tons of product along with regulatory file drafting.
This activity applies equally to generic active ingredients and the ongoing development of novel molecules.
Each chemical development and pharmaceutical phase imposes specific regulatory, analytical and volume-related restrictions which are taken on by M2i, right up to drafting of the regulatory file for the active substance (DMF/ASMF) essential for obtaining marketing authorisation of a drug.
 |
 |